| Fundamentals of water-soluble lubricants |
仠 Preface
1.Dissolution
For easily understanding of water-soluble lubrications, knowledge about character of water molecule is required.
Water molecule formula, (H2O), two hydrogen with one oxygen, is considered as electric charged molecular group of hydrogen ion(H+) and hydroxide ion(OH亅), which is specific liquid, changing by surrounding circumstances(temperature, pressure).
Dissolution phenomenon of some materials in water can be explained that
the materials have also electric chargeable character and the ionic materials
will make combined situation with water ions by pulling power of pair ions(
+ and -).
2.Dispersion
On the other hand, non-electric chargeable materials will not have No.1phenomenon. To dissolve such materials into water, intermediate materials which have the part of water combining character and the part of material combining character are required. Such materials are called emulsifier or surface-acting materials in technical terms. And the system entered into water is called emulsion (soluble or dispersion) and the field of study is surface chemicals. This action is against a law of nature, (handling against law of entropy increase), therefore such a question should be left in energy theory (by virtue of power). Beside physical chemical details, concretely explanation about water-soluble lubricants from viewpoint of emulsion liquid will be done as follow.
仠 Materials for particle dispersion
Stable dispersion of oil into water as small particles can be called emulsification,
and the liquid formed by emulsification is called emulsion. The structure, being
mentioned above 2 in brief, has two characters in the molecules, one is adaptable to water (hydrophilic
group), and the other is estranged to water (hydrophobic group). These two characters
are combined in one structure.
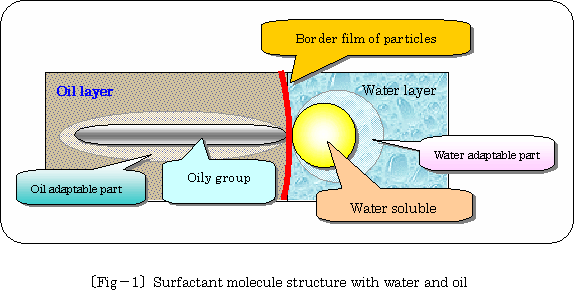
Surfactant molecule structure with water and oil is illustrated enlarged
imaginably as following 乲fig.亅1乴.
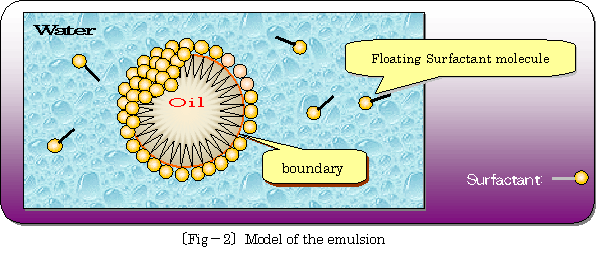
仠 Situation of dispersion of oil surrounded by surfactant molecules in water is illustrated as following 乲fig.亅2乴
仠 Decision of particle diameter of emulsified liquid
Particle diameter depends on quantity of surfactant and quantity of dispersing
oil (surface size), or big quantity of surfactant makes small diameter
while small quantity makes large diameter.
Particle diameter from 0.1乣50 micron can be produced.
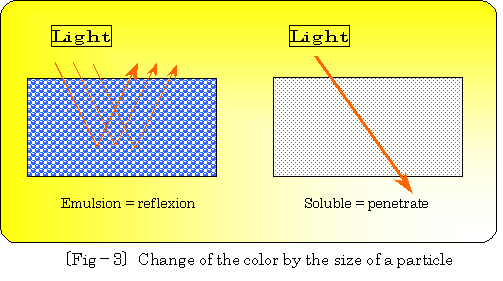
And emulsion can be called white turbidity liquid, reflecting light on
surface of large oil particle(depends wavelength), on the other hand transparent
consecutive phase can be made by passing light through small oil particles.
| Generally, white turbidity is defined as emulsion type, and transparent liquid is defined as soluble type. |
(Particle diameter : emulsion ranges 10乣1.0 , soluble ranges 1乣 0.1micron) Color changing by size of particle diameter (related to wavelength) is imagenablely illustrated as following 乲fig.乗3乴
仠 Problem of dissolved situation
Dissolved situation is how to move the materials dissolved or dispersed
in water. In sticky material like thick malt syrup, particles cannot transfer
or move. On the other hand, high density of particles also makes the same
phenomenon. To prepare easy removable conditions is important for more
effective activity.
仠 Character of emulsion liquid
1. Stability problem of emulsion
Stability factors of emulsion liquid consist of strength (membrane strength)
of border membrane formed on oil particle by surfactant and the diameter
of oil particle, depending on quantity and quality of surfactants.
However, this kind of condition is easily broken by complicated factors
of various physical factors such as temperature, viscosity, specific gravity,
etc. Especially temperature factor gives big effect to break the condition.
Therefore, as so many factors are complicated each other in emulsion liquid
situation, long time stability can乫t be expected.
2. Condition of effective activity in emulsion liquid
The most important aspect in emulsion liquid characters is when, on what
situation the emulsion liquid is formed and how much consistency is best
for effective cleaning and lubrication.
Cleaning effect comes into existence when surfactants adsorb the surface
of dirty materials and form capsule figure.
Therefore, enough quantity to cover whole surface of dirty materials is
required. (less than required quantity would make no effect)
The least required amount is called CMC (critical micelle concentration).
To measure consistency can show situation and direction of emulsion liquid
in question. The measured data can be used for evaluation of lubrication
effectiveness (please confer our company thesis for it),and become startpoint
for analyzing various phenomena .Concrete explanation about meaning of
water-soluble lubricants for big size wires is shown as following 乲fig.亅4乴.
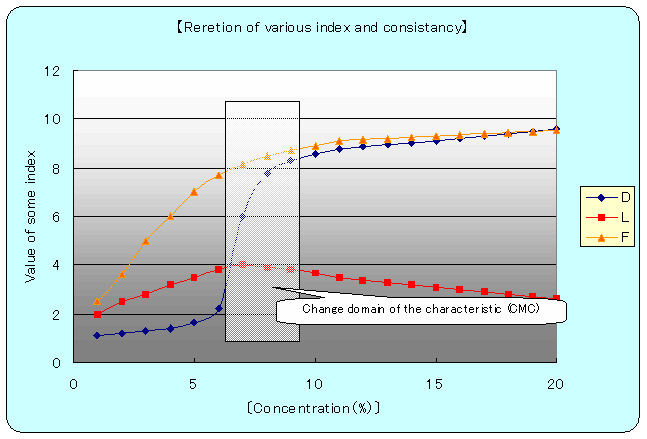
乲fig.亅4乴D=Detargency, L=Lubricity, F=Film intensity
Oil membrane strength curb shows stability of emulsion particles and follows the resemble curb of lubricant efficiency curb. Point to be paid attention is that consistency of 7乣8亾 indicates the biggest figure.
And in cleaning efficiency curb at 6亾 point , drastic changing can be seen and equilibrium situation occurs, at 9亾. As common phenomenon, drastic character changing can be seen at some fixed consistency. Therefore, the more or less consistency of the biggest changing point indicates the emulsion liquid character itself.
仠 Contents of water-soluble lubricants and their effectiveness
Water-soluble lubricants are added functions to above-mentioned formation
of emulsions liquid. Typical contents are shown in following table.
| Contents | Purposes and functions | Types |
|
嘆oil, oily chemicals |
Fundamental materials to separate two phases, and to form the biggest particles(play roll of cushion ball), especially having functional groups and outstanding adsorption power |
|
|
嘇surfactants |
Membrane to form oil particles, changing type according to oil or oil chemicals
species. |
|
| 嘊additives |
1. Prevention of oil chemicals oxidation 2. Breaking surfactants bubbles 3. Prevention of oil and metal deterioration by reacting each others |
1.Stabilizer for anti-oxidation 2.Extinguishing babbles for chemicals |
仠 Movement of formed emulsion followed as upper table, playing cushion ball
like between 2 phases is illustrated as following 乲fig-5乴
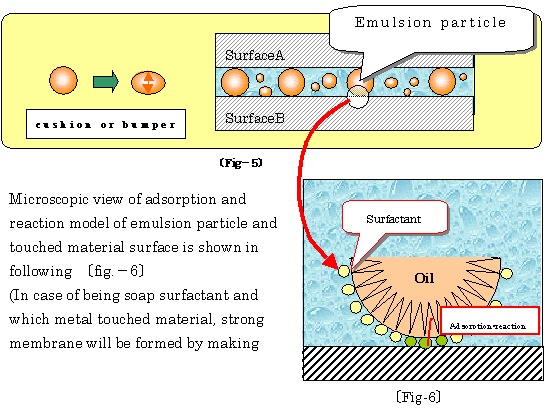
仠 Using purposes and their effects of water-soluble lubricants
Explanation of necessity of using water-soluble lubricants.
1.As water has higher specific heat than straight oil , higher cooling effect for forming heat in metal processing is expected.
2.Noninflammability for safety point of view.
3.Economical because of main contents consisting water.
Above three items are main purposes.
仠 Character of water-soluble lubricants
In case of using water-soluble lubricants, following items should be considered beforehand.